This article originally appeared as an op-ed in The Hartford Courant.
Dr. Ajay Kumar
Chief Clinical Officer
Hartford HealthCare

The list of what we have learned about the virus that causes COVID-19 is a long one. As expected, we’ve gained a fair deal of knowledge regarding immunology and epidemiology — as well as important lessons in psychology and sociology. At the same time, a list of what we don’t know about this virus and its variants would be practically endless.
Scientists are accustomed to dealing with the unknown; confronting mystery and unraveling secrets are among the best parts of the job. But most of us crave certainty, especially when it comes to life-or-death decisions. Questions about the COVID-19 vaccines create hesitancy and fuel resistance — one of the reasons that about 30% of American adults remain unvaccinated.
The Food and Drug Administration can answer one question definitively right now: The mRNA vaccines for COVID-19 are not “experimental.” They are safe, they are effective, and we have nearly 400 million proof points — the number of vaccines administered in the U.S. — to underscore those statements. This extent of scrutiny has never been given to any therapeutic modality in human history and it has proved to be a significant scientific advancement as these vaccines have changed the course of COVID-19 disease.
To be clear, the FDA has never suggested that it unleashed a large-scale medical experiment on American society. But that, perhaps understandably, is how some people interpret the FDA’s “Emergency Use Authorization” classification of the vaccine. Without its stamp of final approval, some Americans believe — or lead others to believe — the vaccine is unstable, unsafe or even dangerous. Whether people believe this sincerely, or if this is simply a convenient excuse for vaccine resistance, is beside the point. It’s an objection the government’s medical-safety watchdog agency can resolve fairly easily.
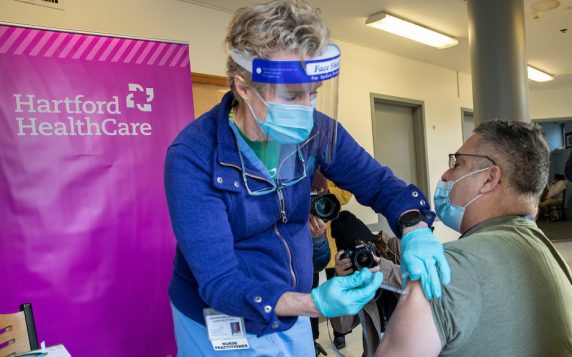
The mRNA vaccines’ paths from labs to jabs has been well-documented. The trials for this medicine were more stringent than for other vaccines, involving 44,000 participants. Even emergency-use authorization was further held up for two more months to gather additional safety data. Every day, since the first U.S. dose was given on Dec. 14, 2020, the FDA has continued to collect data on any possible adverse events. In my health system alone, we have safely administered a half-million doses of vaccine. (Above, Hartford Mayor Luke Bronin receiving a vaccination.) But the uptake is slowing, even as cases rise and the delta variant spreads.
It’s time for the FDA to grant final approval to the Pfizer and Moderna vaccines. This is both a professional and personal issue for me, as a physician and someone who has lost a beloved family member to this disease. The stakes have never been higher as we face new variants and a limited time window to contain this raging pandemic. In addition to acknowledging their already-proven safety, FDA approval would remove one more barrier to promoting vaccination.